number of electrons in bromine|How to find the Number of Protons, Electrons, Neutrons for : Baguio Element Bromine (Br), Group 17, Atomic Number 35, p-block, Mass 79.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main content Based on an assessment of practices and motorcycle-related data, the following are recommended: motorcycle parking stall dimensions of 4.5 ft by 8 ft, 1 motorcycle parking stall per 24, 36, 48 or 60 auto stalls, depending on the State, and an equivalency factor of four motorcycles per auto. To develop a more comprehensive set .
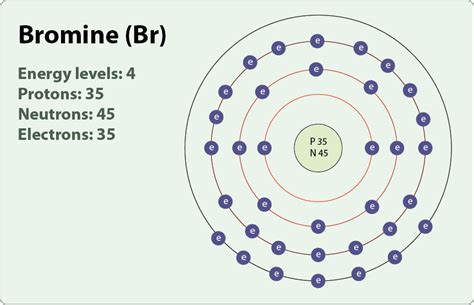
number of electrons in bromine,Element Bromine (Br), Group 17, Atomic Number 35, p-block, Mass 79.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main contentThe atomic number of each element increases by one, reading from left to .The atomic number of each element increases by one, reading from left to .Fifty years ago bromine was produced on a massive scale and turned into lots of .
Bromine is a chemical element with atomic number 35 and 35 electrons in its neutral atom. Learn about its isotopes, electron .
Bromine is the 35th element in the periodic table and has a symbol of Br and atomic number of 35. It has an atomic weight of 79.904 and a mass number of 79. Bromine .
138. 17K views 3 years ago. In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Bromine .Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between .Symbol: Br. Atomic Number: 35. Atomic Mass: 79.904 amu. Melting Point: -7.2 °C (265.95 K, 19.04 °F) Boiling Point: 58.78 °C (331.93 K, 137.804 °F) Number of .Bromine is a nonmetal with atomic number 35 and symbol Br. It has 35 electrons in its neutral state and can form various oxidation states. Learn more about its properties, .
Bromine Electron Configuration: Bromine (Br) is a chemical element. The atomic number of bromine is 35. It is the fuming red-brown liquid at room temperature and the third-lightest halogen. It evaporates . 1st shell can hold 2 electrons. 2nd shell can hold 8 electrons. 3rd shell can hold 18 electrons. 4th shell can hold 32 electrons. Now the atomic number of bromine .Determine the total number of electrons in the valence shells of bromine atoms. The bromine molecule contains only one element. In the periodic table, bromine is a group VIIA element with seven electrons in its last shell. . Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 with the valence electrons in the 4s .
For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence .number of electrons in bromine How to find the Number of Protons, Electrons, Neutrons for A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Therefore, the atomic number also indicates the number of electrons in an atom. The total number of protons and neutrons in an atom is called its mass number (A). The number of neutrons is .
In this video we’ll use the Periodic table and a few simple rules to find the number of protons and electrons for the Bromide ion (S 2-). From the Periodic T.
Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. The chemical symbol for Bromine is Br . Bromine is the third-lightest halogen, and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly coloured gas.Almost all of the mass of an atom is contained within a tiny (and therefore extremely dense) nucleus which carries a positive electric charge and almost all of the volume of an atom consists of empty space in which electrons reside (Figure \(\PageIndex{1}\)). The extremely small mass of the electron (1/1840 the mass of the hydrogen nucleus) causes it to .
Bromine, element symbol Br, has an atomic number of thirty-five. One can find bromine, a halogen, in the p-block, group 17, particularly in period 4. Bromine is between chlorine and iodine, and has reactivity between the two. Bromine’s electron configuration is . The highest principal quantum number is 2. There are 2 electrons in the 2s subshell and 2 electrons in the 2 p subshell, giving carbon a total of four valence electrons. Bromine’s ground state electron configuration is 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 4p 5. The valence electrons are be the 4s and 4p electrons. Bromine has seven .
The bromine atom possesses 35 electrons. It contains 6 electrons in 2p orbital, 6 electrons in 3p orbital and 5 electrons in 4p orbital. Which of these electron experiences the lowest effective nuclear charge?
Total number of electrons of the valance shells of Br 2. There is only one element in bromine molecule. Bromine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of bromine atoms.

Determine the number of protons, neutrons, and electrons in the following isotopes that are used in medical diagnoses: (a) atomic number 9, mass number 18, charge of 1− . Bromine has two isotopes, 79 Br and 81 Br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69% and 49.31%) were determined in earlier experiments. .How to find the Number of Protons, Electrons, Neutrons for Number of Electrons (with no charge): 35; Number of Neutrons (most common/stable nuclide): 45; Number of Protons: 35; Oxidation States: ±1,5; Valence Electrons: 4s 2 p 5 . Bromine - Br (EnvironmentalChemistry.com)- Comprehensive information for the element Bromine - Br is provided by this page including scores of properties, element .Bromine atomic orbital and chemical bonding information. . You may have an easy way to know the number of electrons in a neutral atom, but the placement of those electrons gets a little more complex. Let's take a .
number of electrons in bromineThe atomic number of Bromine Br is 35. The electronic configuration of Bromine Br can be written as 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5; The valence electrons are the sum of the electrons in the outermost shell, that is two 4 s electrons and five 4 p electrons which gives a total of seven valence electrons. Therefore, the valence .
Determine the number of electrons. Protons are particles in the nucleus of an atom that have a positive charge equal to +1. Electrons are particles that have a negative charge equal to -1. Therefore, an element in a neutral state will have the same number of protons and electrons.

The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. A neutral bromine atom would also have 35 electrons. In order for a bromine atom to become a #1-# bromide ion, it would have to gain an additional electron.. Below is the Lewis dot structure for a neutral bromine atom, which has seven valence . Bromine - Bromine has an atomic number of 35 with a symbol of Br. It was first discovered in 1826. In its elemental form, it is the diatomic molecule Br 2. At room temperature, bromine is a reddish- brown liquid. . The number of valence electrons in an atom increases down the group due to the increase in energy levels at progressively .Determine the total number of valence electrons in bromine pentafloride, BrF electrons total number of valence electrons: Identify the molecular geometry of BrF What are the approximate bond angles in BrF,? 90 degrees 109.5 degrees 120 degrees 180 degrees A BrF, molecule is Opolar.
number of electrons in bromine|How to find the Number of Protons, Electrons, Neutrons for
PH0 · How to find the Number of Protons, Electrons, Neutrons for
PH1 · Complete Electron Configuration for Bromine (Br, Br
PH2 · Chemical Elements.com
PH3 · Bromine Electron Configuration (Br) with Orbital Diagram
PH4 · Bromine (Br)
PH5 · Bromine